ca orbital diagram
The ground-state electron configuration of cadmium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2. Three rules governing the formation of orbital diagrams are given.
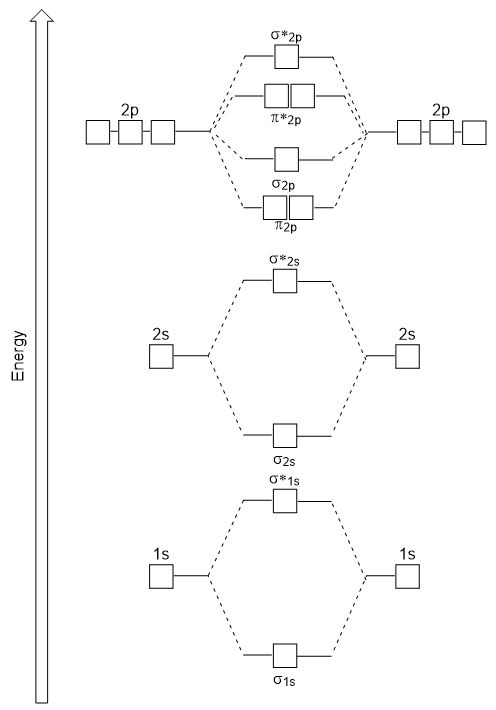
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
Chlorine Orbital Diagram.
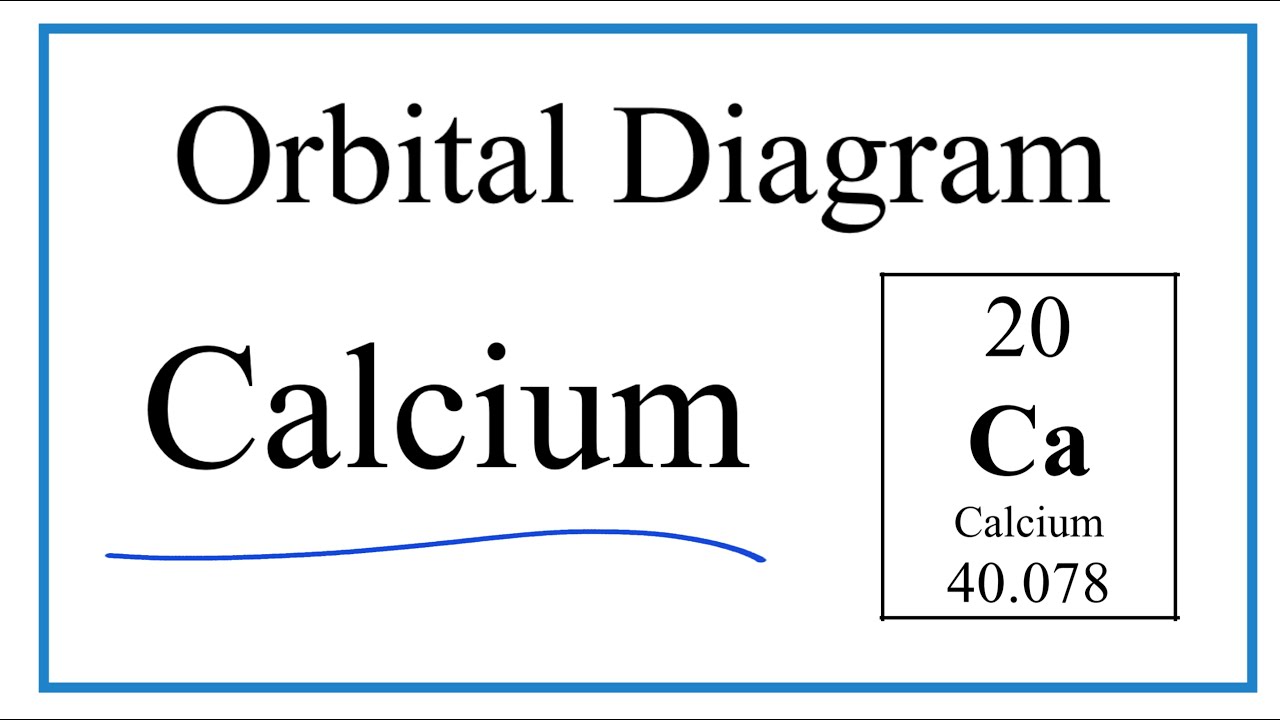
. To write the orbital diagram for the Calcium atom Ca first we need to write the electron configuration for just Ca. The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom it uses an arrow to represent the electrons every orbital one box contains a maximum. The orbital diagram of chlorine shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s subshell has 2 electrons and the 3p subshell has 5 electrons.
It explains how to write the orbital diagram n. View the full answer. Orbital Diagram for CalciumCa Electron configuration of calcium in the excited state.
1s22s22p63s23p64s2 or Ar 4s2. The first one being the Auf Bau. Similar to atomic orbitals we can write electron configuration energy diagrams for molecular orbitals Figure 920 Hydrogen molecular orbital electron configuration energy diagram.
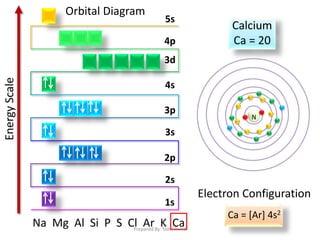
83 6 ratings Calcium has atomic number of 20 so it has total of 20 electrons which should. Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well as giving each orbital its own line to represent the. In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital.
The orbitals are 1s 2s 2p 3s 3p and 4s. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. This is called quantum jump.
Orbital diagrams are a pictorial description of electrons in an atom. Atoms can jump from one orbital to another orbital in an excited state. The total number of electrons in chromium is twenty-four.
Therefore the valence electrons of cadmium are two. Get Ca Orbital Diagram MP3 Download 309 MB on Navidbiglarimusic Quick and Easy - NAVID BIGLARI MUSIC How To Write The Orbital Diagram For Calcium Ca 0215 min 320 kbps 309. Orbital diagrams are known as pictorial descriptions of the electrons in an atom.
To do that we need to find the number of electrons for the. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. Part B Enter an orbital diagram.
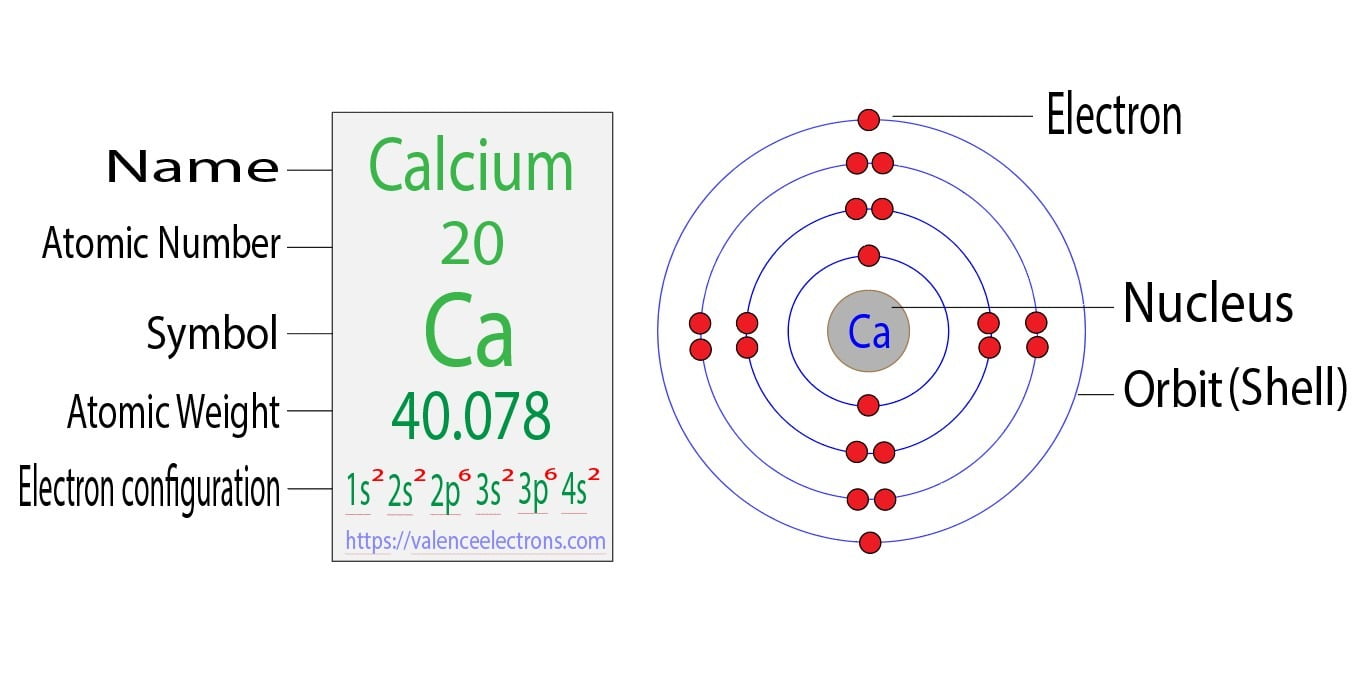
Chromium Cr electron configuration and orbital diagram. This electron configuration shows that the last shell of cadmium has two electrons and the d-orbital has a total of ten electrons. The Calcium orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital the two electrons in.
Chromium is the 24th element in the periodic table and its symbol is Cr. Calcium Ca Number of electrons per shell 2 8 8 2 Number of valence electrons. In order to figure out where electrons go in an atom we have to follow 3 main rules.
Orbital Diagram of All Elements Diagrams.
Chem4kids Com Calcium Orbital And Bonding Info
Chem 2303 Supplementary Problems

Draw An Orbital Diagram For Al Electrons And Ions Which Electrons Are Responsible For Chemical Properties Valence Electrons Core Electrons Ppt Download

2 7 Molecular Orbital Theory Inorganic Chemistry For Chemical Engineers
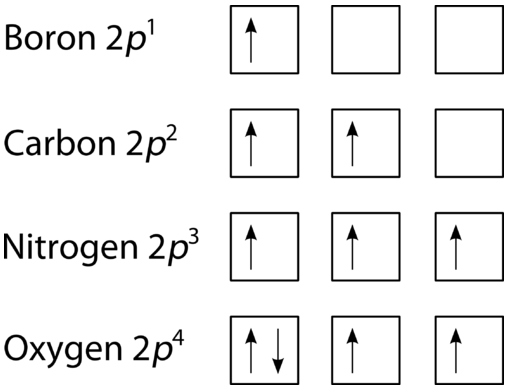
5 17 Hund S Rule And Orbital Filling Diagrams Chemistry Libretexts
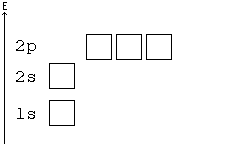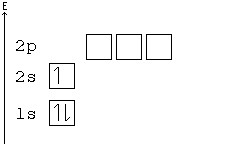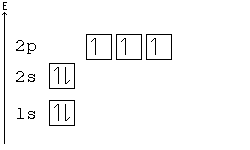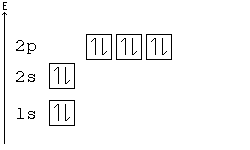
General Chemistry Filling Electron Shells Wikibooks Open Books For An Open World
The Aufbau Principle Video Khan Academy
Calcium Ca Electron Configuration And Orbital Diagram
Calcium Orbital Diagram Free Images At Clker Com Vector Clip Art Online Royalty Free Public Domain
Electronic Configuration

Electron Configuration Wikipedia

Draw The Orbital Diagram Of Calcium 2 Ion Brainly In

Atomic Orbital Diagram Of Calcium 2 Ion Youtube

File Orbital Diagram Nitrogen Hund S Rule Svg Wikimedia Commons
Chem Electron Configuration Diagrams Scientific Tutor

Molecular Orbital Diagram Of Trans Os 4 Ca Cc 6 H 4 Ca Cph Cl Ph 3 4 Download Scientific Diagram

20 Calcium Quicycle Society